The relatively high levels of circulating estrogens (E2) across gestation preclude E2 concentration as a viable direct modulator of the myometrial transition between quiescence and contractility at term. We hypothesize that changes in alternatively spliced Estrogen Receptor alpha (ERα) isoforms across gestation permit uterine specific temporal contractile responses to increasing E2 levels as term approaches. In this study we examined the role of alternately spliced myometrial ERα variants by identifying and knocking down the relevant splice factors utilizing siRNA and lentiviral shRNAs and consequently examining the expression profile of ERα isoform ratios and their downstream targets, the contractile associated proteins (CAPs).
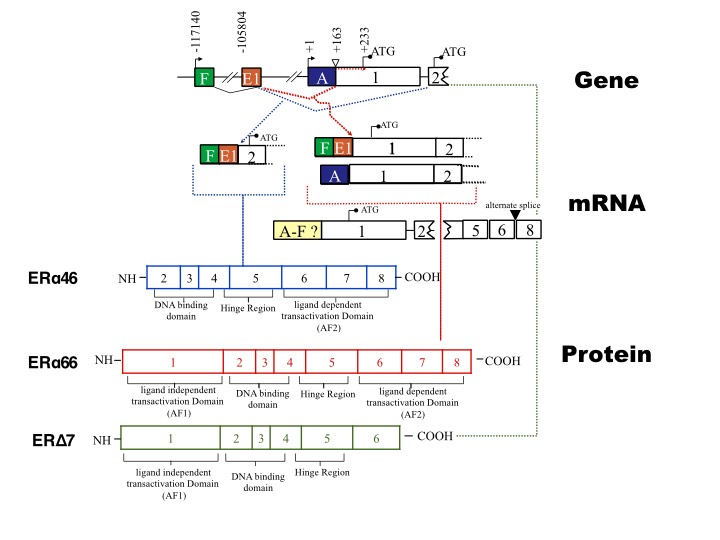
ERα isoforms in term and preterm human myometrium.
Western analysis of the pregnant myometrium shows that ERα66 is present only in the cytoplasm at relatively stable levels (Fig 1A). The nuclear fraction of proteins however shows the presence of ERα46 and ERΔ7 in the nucleus with the 51 kDa (ERΔ7) isoform being lower in term as compared to preterm myometrium (Fig 1A). Using the G-20 ERα antibody from Santa Cruz we see the same pattern of expression in the nucleus with lower levels of expression of the 51 kDa at term (ERΔ7) protein (Fig 1B).
Gestational Changes in Splice Factors involved in alternate splicing.
We are currently carrying out a survey of splice factors during gestation in the mouse. We are particularly interested in SFs that differentially regulated in gestation but can be altered by hormonal input (i.e. P4). We have shown that all SR proteins are expressed at the transcription level during gestation in the myometrium. (Data not shown). We also show that SRSF1 (SF2/ASF data not shown) and hnRNPA1 (Figure 3B) is gestationally regulated, which Pollard et. al (Pollard, A. J., et al. (2000). J Clin Endocrinol Metab 85(5))showed agrees in the human myometrium during human gestation. Finally there is a shift in hnRNPA1 levels when mice were P4 treated (Figure 3B) suggesting hormonal control of this splice factor.
Serum E2 levels rise throughout pregnancy to levels that are 300 times that of a non-pregnant woman. These increasing levels preclude E2 concentrations as a viable direct modulator of myometrial quiescence. We hypothesisze that the three isoforms ERα66, ERα46 and ERΔ7 by virtue of their competitive interactions change CAP protein expression during pregnancy signifying a biochemical mechanism by which estrogens modulate myometrial quiescence. ERα46, lacks an activation function domain 1 (AF-1), is the transcriptionally active form in endothelial cells and osteoblast while ERΔ7 is missing exon 7 and lacks a ligand-binding domain (LBD) is the dominant negative isoform and has been shown to functionally repress the full length ERα and ERb in vitro by dimerization. The interaction of ERα46 with ERα66 may be transcriptionally negative in some instances while being synergistic on other promoters. We have shown the P4 is able to delay ERα66 nuclear localization from E18 to E19 in the mouse and that P4 also advances hnRNPA1 expression from E15 to E17. The alternative splicing of ERα may be under the direct contro of P4.
Our latest work on the dominant negative ERΔ7 has shown that this alternative transcript translates into an estrogen receptor that has a dominant negative role. We beleive that this isoform functions to downregulate GJA1 (connexin 43, a known contractile associated protein) thus keeping the myometrium in a quiescent state early in pregnancy. ERΔ7 decreases as gestation proceeds toward term allowing GJA1 expression through normal estrogen action resulting in a contractile myometrium. We recently published this work in Ebiomedicine (a Lancet Journal.). In this reserach we also show that hnRNPG is the splice factor that generates the exon 7 loss that resuults in ERΔ7 generation and that the levels of hnRNPG are commensurate with the levels of ERΔ7 in the pregnant human myometrium. We also show that in vitro that overexpression of ERΔ7 results in a loss of GJA1 which in turn reduces the contractility of the cells ( as seen in a collagen contractility assay).